The spreading and creeping of oily fluids on the surfaces of solid bodies follows the rule of the cohesion/adhesion ratio between the respective surface tensions of the lubricant and the solid body it is in contact with.
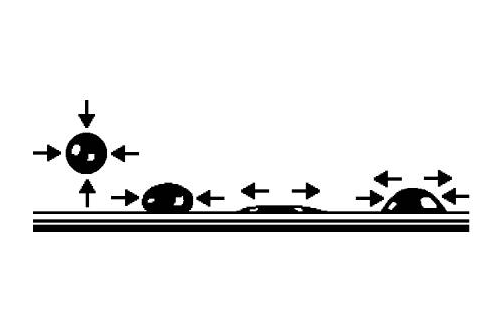
The cohesive force or surface tension of the fluid leads to the drop of oil contracting into a spherical shape, and the adhesive force is the result of the free surface energy of the solid body, and leads to total wetting of the free surface. The requirement for point lubrication brings about an equilibrium between these two forces, but if the adhesive force of the solid body is greater the result will be a wetted surface without any drops forming.
Wetting behaviour
The cause of wetting lies in the physics of the boundary layer behaviour of fluids. The wetting of solid bodies by fluids is influenced by the surface tension of the fluid, the apparent surface tension of the solid body, and by capillary effects.

Capillary effect. Oil rises higher in a narrow capillary than it does in a wide capillary.
Capillary effects on surfaces may be features such as textured scores on the surface caused by machining processes or surface conditions that have been produced deliberately for aesthetic reasons, e.g. a matt finish. Lubricants creep more on rough surfaces than on smooth surfaces.
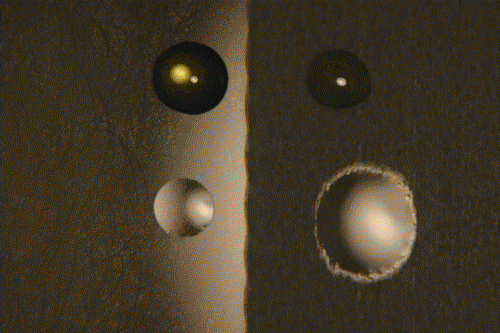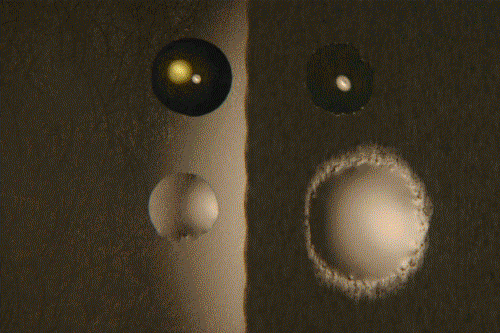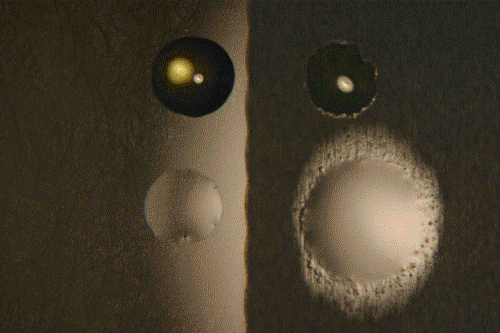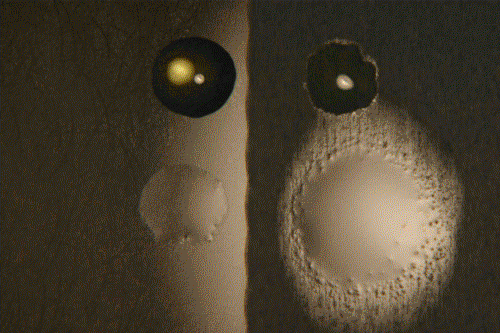
Left, smooth surface, right, rough surface. Above, polyglycol, below, silicone oil.
Time: 1 hr. at 50°C. Surfaces have not been epilamized.
Edges pointing outwards act as oil barriers, edges pointing inwards act as oil repositories. These surface effects can be influenced in the design process relatively easily once the way in which they work has been recognised. The situation with the fluid's wetting behaviour towards the solid body itself is different. From a chemical point of view, increasing the surface tension of a fluid is extremely complicated, indeed in most cases impossible. The additives required to do this need to be added in such high concentrations that they adversely affect the other properties of the lubricant. The solid body, i.e. the structural material itself, cannot be altered in terms of surface tension without affecting its strength characteristics.
Low-viscosity lubricants behave in a striking way at higher temperatures (100°C or above). Wetting speeds in excess of 5 mm per hour can be measured.
